Fuel Cell Materials: Nickel Oxide
In the recent years, nickel oxide is being focused upon as the material for Solid Oxide Fuel Cells (SOFC).
We have received a high evaluation from our customers because of the technology for controlling powder materials that has been cultivated over many years by our business partner and manufacturer of nickel oxide, Sumitomo Metal Mining Co., Ltd. in Japan.
DJK Europe has been certified under REACH to distribute nickel oxide powder in Europe
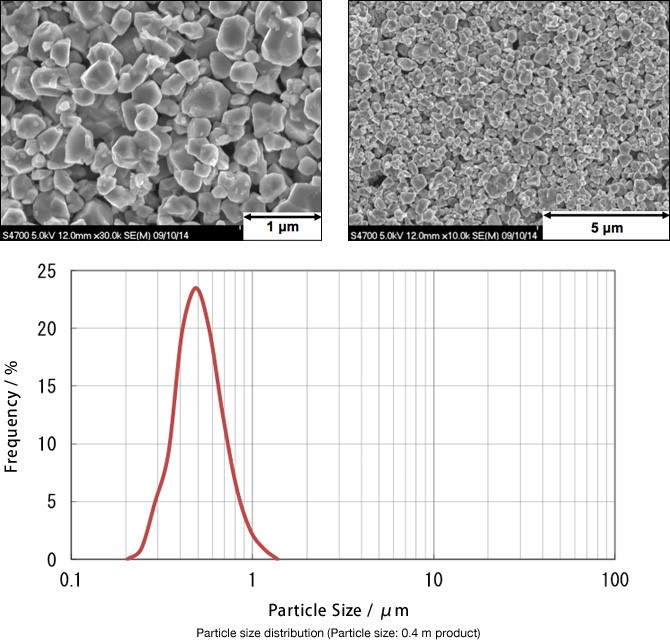
Characteristics
IP grade
- This is a high-purity powder developed through our unique technology.
- This powder has been optimized as an SOFC electrode material.
- Applications
Applications
- Electrodes for Solid Oxide Fuel Cells (SOFC)
- Electrodes for Solid Oxide Electrolysis Cells (SOEC)
- Multilayer inductors
Solid Oxide Fuel Cells (SOFCs)
Solid Oxide Fuel Cells (SOFCs) are high-temperature electrochemical devices that convert chemical energy directly into electricity. They operate by utilizing a solid ceramic electrolyte, typically made of zirconia-based materials mixed with nickel oxides, which conducts oxygen ions at elevated temperatures (typically around 500 to 1000 degrees Celsius). A typical SOFC consists of three main components: the anode, the cathode, and the electrolyte. At the anode, a fuel, such as hydrogen, carbon monoxide, or a hydrocarbon, is oxidized, releasing electrons. At the cathode, oxygen from the air reacts with the electrons and any remaining fuel, generating water vapor as a byproduct.
SOFCs are known for their high energy conversion efficiency, fuel flexibility, and low emissions, making them suitable for various applications, including stationary power generation, residential cogeneration systems, and portable power devices.
Solid Oxide Electrolysis Cells (SOECs)
Solid Oxide Electrolysis Cells (SOECs) are closely related to SOFCs but function in reverse. They operate by using electrical energy to drive the electrolysis of water vapor or carbon dioxide at high temperatures. The same solid ceramic electrolyte is employed in SOECs, allowing the oxygen ions to migrate from the cathode to the anode. When an electric current is applied, the water vapor or carbon dioxide molecules split into oxygen ions and electrons at the cathode. The oxygen ions then travel through the electrolyte, while the electrons flow externally, completing the circuit. At the anode, the oxygen ions recombine with electrons to form oxygen molecules or react with carbon dioxide to produce carbon monoxide or other valuable chemicals.
SOECs offer an efficient method for producing hydrogen, syngas (a mixture of hydrogen and carbon monoxide), and other important chemicals, with the advantage of utilizing excess renewable electricity during periods of low demand. They have potential applications in energy storage, grid balancing, industrial processes, and fuel production for transportation.
In both Solid Oxide Fuel Cells (SOFCs) and Solid Oxide Electrolysis Cells (SOECs), our high-quality nickel oxide powders play a crucial role as catalyst materials mainly in the anode, facilitating efficient electrochemical reactions and enhancing conductivity for the conversion of chemical energy into electricity or the production of hydrogen and valuable chemicals.
Contact
Our team can support you with price information, product information and live demonstrations.
frankfurt@djkeurope.com
Phone: +49 6196 77614-11
Fax: +49 6196 77614-19

